The answer for the following problem is mentioned below.
- Therefore the volume of potassium chloride solution is 11.8 litres.
Step-by-step explanation:
Given:
molarity of the potassium chloride solution (m) = 0.7 M
no of moles present in the potassium chloride solution (n) = 8.30 moles
To find:
volume of the potassium chloride solution (V)
We know;
m =
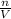
Where;
m represents the molarity of potassium chloride solution
n represents the no of moles of potassium chloride solution
V represents the volume of the solution
So;
0.7 =
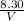
V = 11.8 litres
Therefore the volume of potassium chloride solution is 11.8 litres.