The volume of a balloon f a gas at 842 mm Hg and -23 celsius if it’s volume is 915 milliliters at a pressure of 1170 mm Hg And a temperature of 24 celsius is 0.22 litres
Step-by-step explanation:
Data given:
Initial volume of the balloon having gas V1= 915ml OR 0.195 L
initial pressure of the gas P1= 1170 mm Hg OR 1.53 atm
initial temperature of the gas T1 = 24 celsius or 273.15 + 24 = 297.15 K
Final pressure of the gas P2 = 842 mm Hg or 1.10 atm
final temperature of the gas T2 = -23 degrees or 273.15 - 23 = 250.15 K
Final volume at final temperature and pressure V2=?
The formula used is of Gas Law:
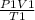 =
=
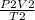
V2 =
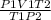
putting the values in the equation:
V2 =
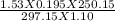
V2 = 0.22 litres is the volume
The volume is 0.22 litres at a pressure of 1170 mmHg and temperature of -23 degrees.