Balanced chemical equation for the reaction is:
2S
 (g) +
(g) +
 (g)+ 2
(g)+ 2
 O (l) ⇒
O (l) ⇒
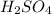
Moles of
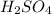 formed is 5.75 moles.
formed is 5.75 moles.
Moles of oxygen used is 5.75 moles in the reaction.
Step-by-step explanation:
Data given:
moles of S
 = 11.5 moles
= 11.5 moles
moles of
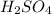 = ?
= ?
Moles of
 needed =?
needed =?
balanced equation with states of matter =?
Balanced chemical reaction under STP condition is given as:
2S
 (g) +
(g) +
 (g) + 2
(g) + 2
 O (l) ⇒
O (l) ⇒
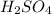
From the balanced reaction 2 moles of sulphur dioxide reacted to form 1 mole of sulphuric acid:
so, from 11.5 moles of S
 , x moles of
, x moles of
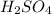 is formed
is formed
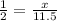
2x = 11.5
x = 5.75 moles of sulphuric acid formed.
From the balanced reaction 1 mole of oxygen reacted to form 1 mole of sulphuric acid.
when 11.5 moles of Sulphur dioxide reacted then oxygen in the reaction is 5.75 moles.