Answer:
the temperature of the non- catalyzed reaction is = 2793.75 K
Step-by-step explanation:
The reaction of the spontaneous decomposition of hydrogen peroxide to give water and oxygen is given as:
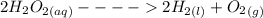
The activation energy of non-catalyzed reaction
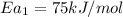
The activation energy of metal catalyzed reaction
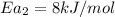
The temperature of metal catalyzed reaction
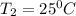 = (25+273)K = 298 K
= (25+273)K = 298 K
The rate constant of the non-catalyzed reaction can be expressed as:
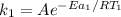 ----- equation (1)
----- equation (1)
The rate constant of the metal catalyzed reaction can be expressed as:
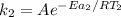
Then
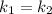
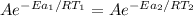
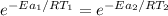
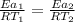
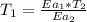
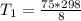
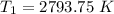
Thus; the temperature of the non- catalyzed reaction is = 2793.75 K