The new volume of the dilute solution is 0.33 L.
Step-by-step explanation:
Using the law of Volumetric analysis, we can find the volume of the dilute solution from the stock solution or the concentrated solution of Calcium Chloride.
V1M1 = V2M2
V1 be the volume of the stock solution = 0.25 L
M1 being the molarity of the stock solution = 0.98 M
V2 be the volume of the dilute solution = ?
M2 being the molarity of the dilute solution = 0.74 M
We have to rearrange the above equation to find V2 as,
V2 =
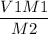
Now plugin the values as,
V2 =
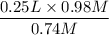
= 0.33 L
So the new volume of the dilute solution is 0.33 L.