Answer:
About 3.81 grams.
Step-by-step explanation:
We can use the ideal gas law. Recall that:
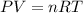
Note that the universal gas constant R has the value 0.08206 L-atm/mol-K.
Hence, convert the measured pressure to atms (1 atm = 14.7 psi):
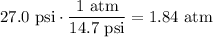
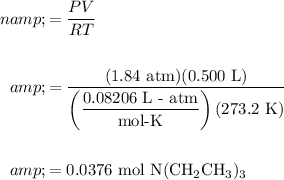
Rearrange the equation to solve for n, the number of moles of vaporized triethylamine and evaluate. The temperature is (25.0 + 273.15 ) K = 298.2 K:
Convert from moles to grams:
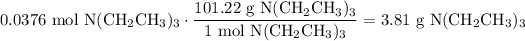
In conclusion, there is about 3.81 grams of vaporized triethylamine in the lecture bottle.