The volume of Potassium chloride solution is 4.8 L.
Step-by-step explanation:
Molarity is found by dividing the number of moles of the given substance by its volume in litres, and so the unit of molarity is mol/L.
Here number of moles is given as 3.34 mol
Molarity = 0.7 M
Volume = ? L
Molarity =
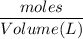
To find the volume we have to rearrange the equation as,
Volume (L) =
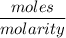
Now, we have to plug in the values as,
Volume (L) =
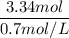
= 4.77 ≈ 4.8 L
So the volume of Potassium chloride solution is 4.8 L.