Answer:
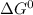 = -457.9 kJ and reaction is product favored.
= -457.9 kJ and reaction is product favored.
Step-by-step explanation:
The given reaction is associated with 2 moles of

Standard free energy change of the reaction (
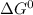 ) is given as:
) is given as:
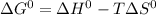 , where T represents temperature in kelvin scale
, where T represents temperature in kelvin scale
So,
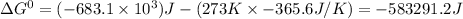
So, for the reaction of 1.57 moles of
 ,
,
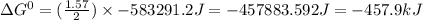
As,
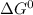 is negative therefore reaction is product favored under standard condition.
is negative therefore reaction is product favored under standard condition.