Question:
The information given is:
Trial # Tiwater T f ΔT Masswater (m)
#1: 21.2 10.8 10.8 24.990
#2: 20.8 9.50 9.5 25.000
#3: 20.9 9.20 9.2 25.010
Answer:
The heat of the reaction is -5985 J
Step-by-step explanation:
The heat absorbed by the water is given by
ΔQ = m·c·ΔT
From which
∑ (ΔT·m)/3 = 278.34 kg·°C
ΔQ = c×∑ (ΔT·m)/3 = 4.184 J/g·°C×278.34 kg·°C = 1164.565 J
ΔQ Calorimter = Specific heat capacity of calorimeter,
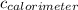 × ΔT
× ΔT
Where the
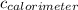 = 443 J/°C for example, we have
= 443 J/°C for example, we have
ΔQ Calorimter = 443×11.133 = 4820.733 J
From which the heat of reaction is then
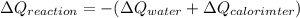
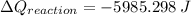
Using 4 digits, we get
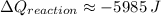 .
.