5.58 X
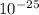 Litres is the volume, in liters, occupied by 0.015 molecules of oxygen at STP.
Litres is the volume, in liters, occupied by 0.015 molecules of oxygen at STP.
Step-by-step explanation:
Data given:
molecules of oxygen = 0.015
number of moles of oxygen =?
temperature at STP = 273 K
Pressure at STP = 1 atm
volume = ?
R (gas constant) = 0.08201 L atm/mole K
to convert molecules to moles,
number of moles =
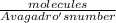
number of moles = 2.49 x
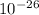
Applying the ideal gas law since the oxygen is at STP,
PV = nRT
rearranging the equation:
V =
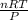
putting the values in the rearranged equation:
V =
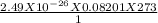
V = 5.58 X
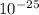 Litres.
Litres.