Answer: A. unsaturated.
Step-by-step explanation:
Unsaturated solution is defined as the solution in which more solute particles can be dissolved in the solvent.
Saturated solution is defined as the solution in which no more solute particles can be dissolved in the solvent.
Supersaturated solution is defined as the solution in which more amount of solute particles is present than the solvent particles.
Given: Solubility = 30g/100ml
If 100 ml can dissolve ionic compound = 30 g
300 ml can dissolve ionic compound =
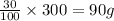
Thus solubility is 90g/300 ml and dissolved salt is only 70 g , the solution is said to be unsaturated.