Answer:
82.18% is the mass percentage of bromine in the original compound.
Step-by-step explanation:
Mass of AgBr = 1.1166 g
Moles of AgBr =
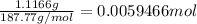
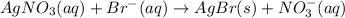
According to reaction, 1 mole of AgBr is obtained from 1 mole of bromide ions , then 0.0059466 moles of AgBr will be formed from :
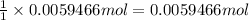 of bromide ions
of bromide ions
Mass of 0.005939 moles of bromide ions :
0.0059466 mol × 79.90 g/mol = 0.4751 g
Mass of the sample = 0.5781 g
Mass percentage of bromine in sample :
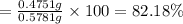
82.18% is the mass percentage of bromine in the original compound.