None of the given options are correct, and the correct answer is 174 K.
Step-by-step explanation:
Mass of N = 41.6 g
Moles =
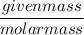
=
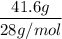 = 1.5 moles
= 1.5 moles
Pressure, P = 815 mm Hg = 1.07 atm
Volume, V = 20 L
R = gas constant = 0.08205 L atm mol⁻¹ K⁻¹
Temperature, T = ? K
We have to use the ideal gas equation,
PV = nRT
by rearranging the equation, so that the equation becomes,
T =
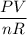
Plugin the above values, we will get,
T =
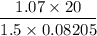
= 174 K
So the temperature of the nitrogen gas is 174 K.