Answer:
Thus, one gram of Hemoglobin carries 1.389 mL of oxygen.
Step-by-step explanation:
Each hemoglobin molecules carries 4 oxygen molecules.
Given that the molar mass of hemoglobin = 64,5000 g/mol
1 gram of hemoglobin =
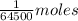
Now, no of moles of oxygen carried in 1 mole of hemoglobin =
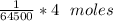
=
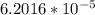 moles
moles
assuming the oxygen is to be an ideal gas; then:
PV = nRT
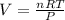
Given that :
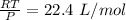
Then V =
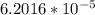
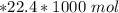
V = 1.389 mL
Thus, one gram of Hemoglobin carries 1.389 mL of oxygen.