Answer:
392.20K
Step-by-step explanation:
-We apply Charles' Law which states that for an ideal gas at constant pressure, its volume is directly proportional to it's temperature:
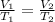
-Given that:
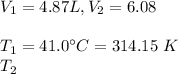
#We substitute in the ratio formula above and calculate for
 :
:
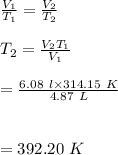
Hence, the temperature of the balloon at a volume of 6.08L is 392.20K