Answer:
SEPARATION SCHEME FOR CATIONS
GIVEN CATIONS :
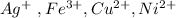
Step 1: Add
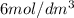 of
of
 to the mixture solution
to the mixture solution
Result : This would cause a precipitate of
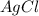 to be formed
to be formed
Reaction :

Step 2 : Next is to remove the precipitate and add
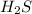 to the remaining
to the remaining
solution in the presence of
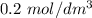 of HCl
of HCl
Result : This would cause a precipitate of
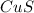 to be formed
to be formed
Reaction :

Step 3: Next remove the precipitate then add
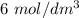 of aqueous
of aqueous
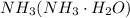 , process the solution in a centrifuge,when the
, process the solution in a centrifuge,when the
process is done then sort out the precipitate from the solution
Now this precipitate is
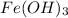 and the remaining solution
and the remaining solution
contains
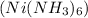
Next take out the precipitate to a different beaker and add HCl
to it this will dissolve it, then add a drop of
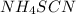 this will
this will
form a precipitate
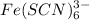 which will have the color of
which will have the color of
blood indicating the presence of

Reaction :



Now the remaining mixture contains
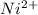
Step-by-step explanation: