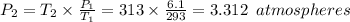Answer:
Step-by-step explanation:
Here we have the mass of CO₂ added = 340 g
From
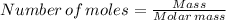
We have, where the molar mass of CO₂ is 44.01 g/mol
Therefore,
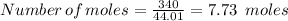
71. Included drawing attached
72. Here we have the pressure of the gas given by Charles law which can be resented as follows;
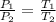
Where:
P₁ = Initial pressure = 6.1 atmospheres
P₂ = Final pressure
T₁ = Initial Temperature = 293 K
T₂ = Initial Temperature = 313 K
Therefore,