Answer:
The concentration is 0.111 M.
Step-by-step explanation:
Use dimensional analysis to solve the problem. Since both HCl and NaOH are strong, the dissociate completely in solution.
For this neutralization, the reaction is HCl + NaOH = NaCl + HOH
18.5 mL x
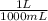 x
x
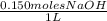 x
x
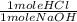 = 0.002775 moles HCl
= 0.002775 moles HCl
Molarity = moles/ Liters
Molarity of HCl = 0.002775 moles HCl / 0.025 L = 0.111 M