Answer:
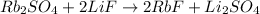
Step-by-step explanation:
In this problem, we have the equation of a chemical reaction to complete.
The uncomplete equation is:
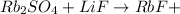
In order to complete the equation, the missing compound must include the missing elements, which are:
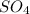
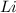
So the missing compound must be
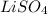
So now we can write:
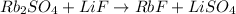
However, the reaction is not balanced yet, in fact we see that:
- We have 2 atoms of Rb on the left, and 1 atom only on the right
So we add a 2 in front of RbF:
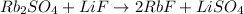
However, now the F is not balanced, so we also add a 2 in front of LiF:
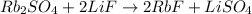
Finally, we see now that the Li is not balanced, therefore:
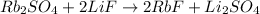
So now the equation is balanced.