Answer:
Approximately
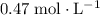 (note that
(note that
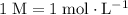 .)
.)
Step-by-step explanation:
The molarity of a solution gives the number of moles of solute in each unit volume of the solution. In this
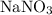 solution in water,
solution in water,
Let
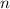 be the number of moles of the solute in the whole solution. Let
be the number of moles of the solute in the whole solution. Let
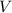 represent the volume of that solution. The formula for the molarity
represent the volume of that solution. The formula for the molarity
 of that solution is:
of that solution is:
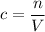 .
.
In this question, the volume of the solution is known to be
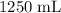 . That's
. That's
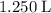 in standard units. What needs to be found is
in standard units. What needs to be found is
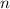 , the number of moles of
, the number of moles of
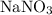 in that solution.
in that solution.
The molar mass (formula mass) of a compound gives the mass of each mole of units of this compound. For example, the molar mass of
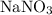 is
is
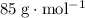 means that the mass of one mole of
means that the mass of one mole of
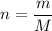 .
.
For this question,
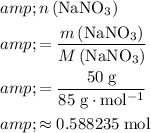 .
.
Calculate the molarity of this solution:
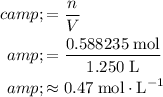 .
.
Note that
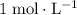 (one mole per liter solution) is the same as
(one mole per liter solution) is the same as
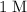 .
.