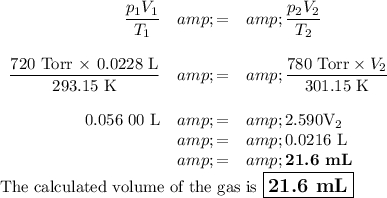Answer:
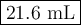
Step-by-step explanation:
We can use the Combined Gas Laws to solve this problem
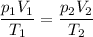
Data
p₁ = 720 Torr; V₁ = 0.0228 L; T₁ = 20.0 °C
p₂ = 780 Torr; V₂ = ?; T₂ = 28.0 °C
Calculations
(a) Convert the temperatures to kelvins
T₁ = (20.0 + 273.15) K = 293.15 K
T₂ = (28.0 + 273.15) K = 301.15 K
(b) Calculate the new volume