0.027 moles of
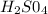 if there are 1.64x
if there are 1.64x
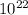 molecules of
molecules of
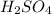
Step-by-step explanation:
Data given:
number of molecules of
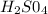 = 1.64 X
= 1.64 X
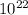
Atomic weight of the sulphuric acid = 98.07 grams/mole
We know that 1 mole of sulphuric acid has 6.022 x
 (Avagadro number) molecules.
(Avagadro number) molecules.
so number of moles present in 1.64 X
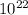 molecules is given by:
molecules is given by:
so to get moles we will use
number of moles =
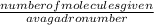
number of moles =
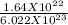
number of moles = 0.027 moles
So, 1.64 X
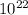 molecules will be there in 0.027 moles of sulphuric acid.
molecules will be there in 0.027 moles of sulphuric acid.