The volume of the drum after chilled quickly is 3.6 L.
Step-by-step explanation:
As per the Charles Law, for any gas at constant pressure, volume of the gas is in direct proportion with the temperature measured in Kelvin. As the temperature increases, the gas expands and vice versa.
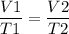
After chilling the drum, the volume will get reduced and it can be found as,
V2 =
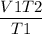
=
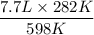
= 3.6 L
Here the drum is chilled quickly, that is temperature is reduced and so the volume also decreases.