Answer:
7.53*10^23 atoms
Step-by-step explanation:
Using stoichiometry convert moles of silver to moles of copper. Based on the equation, for every 2 moles of silver, 1 mole of copper is produced.
Then, convert moles to atoms with Avogadro's number.
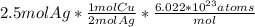
Your answer should be 7.53*10^23 atoms.