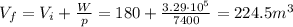Answer:
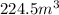
Step-by-step explanation:
By using the first law of thermodynamics, we can find the work done by the gas:
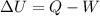
where in this problem:
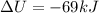 is the change in internal energy of the gas
is the change in internal energy of the gas
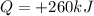 is the heat absorbed by the gas
is the heat absorbed by the gas
W is the work done by the gas (positive if done by the gas, negative otherwise)
Therefore, solving for W,
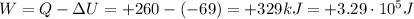
So, the gas has done positive work: it means it is expanding.
Then we can rewrite the work done by the gas as
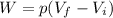
where:
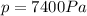 is the pressure of the gas
is the pressure of the gas
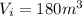 is the initial volume of the gas
is the initial volume of the gas
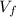 is the final volume
is the final volume
And solving for Vf, we find