48.70 is the volume, in liters, occupied by 1.73 moles of Nitrogen gas at 0.992 atm pressure and a temperature of 75º C.
Step-by-step explanation:
Data given:
number of moles of nitrogen gas = 1.73 moles
pressure of the gas = 0.992 atm
temperature of the gas = 75 degrees or 273.25 + 75 = 348.15 K
R (gas constant) = 0.08206 L atm/molesK
volume of the nitrogen gas under these conditions =?
The formula used is of ideal Gas Law:
PV = nRT
Rearranging the equation to get,
V =
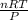
putting the values in the equation:
V =
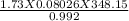
= 48.70 Litres
the volume of the gas is 48.70 litres from the standard values used in the equation.