Answer: 1. n
2. n
3.
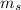
4. l
Step-by-step explanation:
Principle Quantum Numbers : It describes the size of the orbital and the energy level. It is represented by n. Where, n = 1,2,3,4....
Azimuthal Quantum Number : It describes the shape of the orbital. It is represented as 'l'. The value of l ranges from 0 to (n-1).For l = 0,1,2,3... the orbitals are s, p, d, f...
Magnetic Quantum Number : It describes the orientation of the orbitals. It is represented as
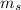 . The value of this quantum number ranges from -l to +l.
. The value of this quantum number ranges from -l to +l.
Spin Quantum number : It describes the direction of electron spin. This is represented as s.