Answer:
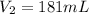
Step-by-step explanation:
Hello,
In this case, Avogadro's relationship allows us to relate the moles and the volume of a gas at an initial condition and an a final one as shown below:
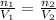
Thus, the volume that 0.00684 mol of neon the same pressure and temperature is computed below as required:
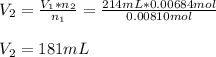
Best regards.