30.26 grams of N
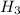 is produced when 5.40 g of
is produced when 5.40 g of
 , is reacted with excess nitrogen gas.
, is reacted with excess nitrogen gas.
Step-by-step explanation:
Data given:
mass of ammonia N
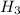 = ?
= ?
mass of Hydrogen gas = 5.40 grams
Balanced chemical reaction:
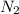 + 3
+ 3
 ⇒2N
⇒2N
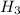
Atomic mass of hydrogen = 2.06 gram/mole
atomic mass of nitrogen = 28.006 grams/mole
atomic mass of ammonia = 17 grams/mole
moles of hydrogen provided
number of moles =

putting the values in the equation:
number of moles=
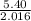
number of moles = 2.67 moles of hydrogen gas is given
from the chemical reaction:
3 moles of H2 gives 2 moles of NH3
SO, 2.67 moles of H2 will give x moles of NH3
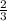 =
=
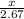
3x = 5.34
x = 1.78 moles of ammonia
mass of ammonia = number of moles x atomic mass
mass of ammonia = 1.78 x 17
mass of ammonia = 30.26 grams