Answer:
2076.9 J/kg°C
Step-by-step explanation:
The equation needed here is:
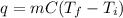 , where q is the energy in Joules, m is the mass in kg, C is the heat capacity, T_f is the final temperature, and T_i is the initial temperature.
, where q is the energy in Joules, m is the mass in kg, C is the heat capacity, T_f is the final temperature, and T_i is the initial temperature.
Here, we have q = 6.75*
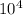 J, m = 1.30kg, T_f = 57, and T_i = 32. So:
J, m = 1.30kg, T_f = 57, and T_i = 32. So:
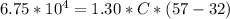
Solving for C, we get:
C = 2076.9 J/kg°C
Hope this helps!