The volume of the balloon at that location is 615 L.
Step-by-step explanation:
We have to find the volume of the balloon, using the volume, pressure and temperature using the equation as,
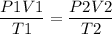
P1 = 0.995 atm
P2 = 0.720 atm
V1 = 5 × 104 L = 520 L
T1 = 32 + 273 = 305 K
T2 = -12+ 273 = 261 K
We need to find V2 by rearranging the above equation as,
V2 =
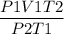
Now plugin the values as,
V2 =
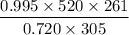
= 614.9 ≈ 615 L
So the volume of the balloon at that location is 615 L.