The answer for the following problem is mentioned below.
Step-by-step explanation:
Given:
mass of iron (m) = 15.75 grams
heat (q) = 1097 J
initial temperature (
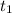 ) = 25°C
) = 25°C
final temperature (
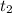 ) = 177°C
) = 177°C
To find:
specific heat (c)
We know;
c = q ÷ mΔT
where;
c represents the specific heat
q represents the heat
m represents the mass
t represents the temperature
c =
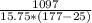
c = 0.45 J/kg°C
Therefore the specific heat capacity of iron is 0.45 J/kg°C.