0.023 moles s equivalent to 5.98 g of K Al (SO₄)₂.
Step-by-step explanation:
Data given:
mass of K Al (SO₄)₂ = 5.98 grams
atomic mass of 1 mole K Al (SO₄)₂ =258.25 grams/mole
39 + 27+ (96.0 x3)= 258.25 grams (K=39 grams/mole, Al = 27 grams/mole, SO4 is 96 grams/mole)
To determine the number of moles the formula used is:
number of moles =

when the values are put in the equation we get the Number of moles the compound has in it in the given mass of that compound.
So,
number of mole=
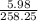
= 0.023 moles
Thus we see that 5.98 grams of K Al (SO₄)₂ is equal to 0.023 moles of it.