50 ml will be the new volume of the carbon dioxide gas in your coke can.
Step-by-step explanation:
Data given:
Initial volume of the coke having CO2, V1 =25 ml
initial pressure of the coke having C
 , P1 = 100KPa OR 0.98 atm
, P1 = 100KPa OR 0.98 atm
final pressure when hiked to Mount Everest P2 = 50 kPa or 0.49atm
final volume V2 when hiked to Mount Everest = ?
From the data given formula for Boyles' Law will be used as:
P1V1 = P2V2
V2 =
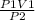
putting the values in the equation, we get,
V2 =
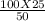
= 50 ml
As we hike on the mountain the drop in pressure causes the volume of gas to increase hence the volume of carbon dioxide gas increased from 25 ml to ml.