160 ml of 3.0 M phosphoric acid (H3PO4) can be made from 95mL of a 5.0M H3PO4 solution. In the option nearest value given is 160 ml. So first option is the right answer.
Step-by-step explanation:
Data given:
Initial volume of phosphoric acid given = 95 ml
initial molarity of the solution of phosphoric acid = 5 M
final volume of phosphoric acid = ?
final molarity to be achieved = 3 M
the formula used for dilution is :
Minitial x V initial = M final x Vfinal
Vfinal =
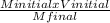
V final =
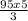
= 158. 33 ml
The solution having more molarity is concentrated here initial solution was concentrated it was diluted to be 3M by taking 158.33 ml of the 5M solution.
From the options nearest value is 160 ml