Answer:
127.013 C
Step-by-step explanation:
-We apply Faraday's First Law of Electrolysis which states that the mass,m of a substance deposited is directly proportional to the current,Q passed through it:
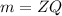
Where Z is the proportionality constant(g/C)
-The current can therefore be calculated as:
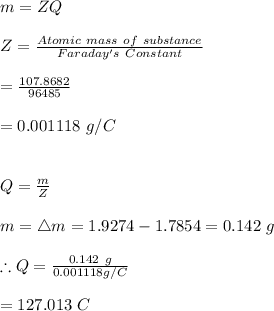
Hence, the amount of current through the circuit is 127.013 C