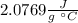Answer:
Step-by-step explanation:
-Specific heat capacity is given by the formula:
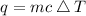
Where:
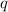 is the heat gained or loosed by the substance
is the heat gained or loosed by the substance
 is the mass of the substance
is the mass of the substance
 is the specific heat of the substance
is the specific heat of the substance
 is the change in temperature
is the change in temperature
#We make c the subject of the formula and substitute to solve for it:
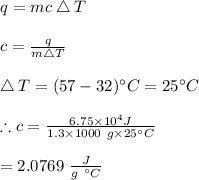
Hence, the specific heat capacity of the ice is