Answer:
When put into steam
Step-by-step explanation:
When a certain amount of steam at boiling temperature condenses (turning into water), the amount of heat released is
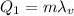
where in this case
m = 25.0 g = 0.025 kg is the mass of steam at 100.0°C
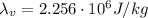 is the latent heat of vaporization of water
is the latent heat of vaporization of water
So,
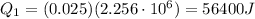
Instead, the amount of heat released when the water at 100.0°C is cooled down to 34.0°C is given by
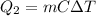
where
m = 25.0 g = 0.025 kg is the mass of water
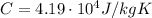 is the specific heat of water
is the specific heat of water
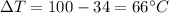 is the change in temperature
is the change in temperature
Therefore,
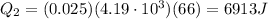
Since
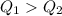 , we can say that your hand will burn more in the first case.
, we can say that your hand will burn more in the first case.