The new volume of the gas is 29.6 L.
Step-by-step explanation:
Using the ideal gas equation, we can find the new volume of the gas y cancelling the gas constant and number of moles as both are same in both the cases.
By using the equation,
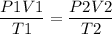
P1, V1 and T1 are the initial values of Pressure, Volume and temperature of the gas and P2,V2 and T2 are the final values of Pressure, Volume and temperature of the gas.
We have to find the new volume by rearranging the equation as,
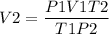
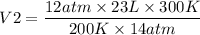
= 29.6 L
So the new volume of the gas is 29.6 L.