Answer:
0.03 mol H₂
Step-by-step explanation:
In a diprotic acid titration, the first equivalence point relates to the equilibrium:
And the second equivalence point to:
We can add those two equations and we're left with:
So to calculate the moles of H₂ that were in the original acid solution we use the total volume used (in this case 600 mL).
600 mL ⇒ 600/1000 = 0.6 L
We calculate the moles of LiOH, using its molar concentration:
- 0.1 M * 0.6 L = 0.06 mol LiOH
And now we convert moles of LiOH (or OH⁻) to moles of H₂ using the stoichiometric ratio:
- 0.06 mol LiOH *
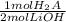 = 0.03 mol H₂
= 0.03 mol H₂