Answer : The partial pressure of X and Y gases are, 0.525 and 1.575 atm respectively.
Explanation : Given,
Moles of X = 2.0 mole
Moles of Y = 6.0 mole
Total pressure = 2.1 atm
Now we have to calculate the mole fraction of X and Y.
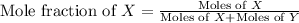
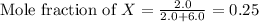
and,
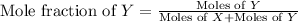
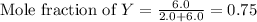
Now we have to calculate the partial pressure of X and Y.
According to the Raoult's law,
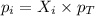
where,
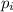 = partial pressure of gas
= partial pressure of gas
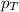 = total pressure of gas = 2.1 atm
= total pressure of gas = 2.1 atm
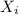 = mole fraction of gas
= mole fraction of gas
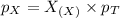
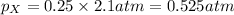
and,
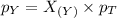
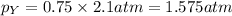
Thus, the partial pressure of X and Y gases are, 0.525 and 1.575 atm respectively.