Answer:
40 g/mol, Argon
Step-by-step explanation:
We can find the number of moles of the gas by using the equation of state for an ideal gas:
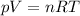
where
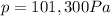 is the pressure of the gas at STP
is the pressure of the gas at STP
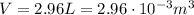 is the volume of the gas
is the volume of the gas
n is the number of moles
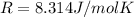 is the gas constant
is the gas constant
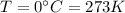 is the absolute temperature of the gas at STP
is the absolute temperature of the gas at STP
Solving for n, we find:
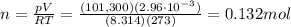
Now we can find the molar mass of the gas, which is given by
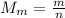
where
m = 5.28 g is the mass of the gas
n = 0.132 mol is the number of moles
Substituting,
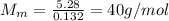
So, the gas in this problem is Argon, which has a molar mass of 40 g/mol.