Answer:
100 grams,

Step-by-step explanation:
Note: I assume you want to find the volume of the gas, since the mass (100 grams) is already given.
First of all, we have to find the number of moles of the gas. This is given by:
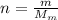
where
m = 100 g is the mass of the gas
 is the molar mass of helium
is the molar mass of helium
Substituting,

Now we have to find the volume of the gas. We can do it by using the equation of state for an ideal gas:
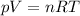
where
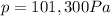 is the pressure of the gas at STP
is the pressure of the gas at STP
V is the volume of the gas
n = 25 mol is the number of moles
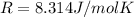 is the gas constant
is the gas constant
 is the temperature at STP
is the temperature at STP
Solving for V, we find the volume:
