Answer:
Approximately
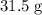 .
.
Step-by-step explanation:
The mass of a solution can be divided into two parts:
- the solute (the material that was dissolved,) and
- the solvent.
In this particular
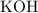 solution in water,
solution in water,
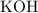 is the solute, while
is the solute, while- water is the solvent.
The number
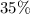 here likely refers to the concentration of
here likely refers to the concentration of
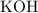 in this solution. That's ratio between the mass of the solute (
in this solution. That's ratio between the mass of the solute (
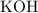 ) and the mass of the whole solution (mass of solute plus mass of solvent.) That is:
) and the mass of the whole solution (mass of solute plus mass of solvent.) That is:
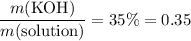 .
.
Hence,
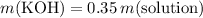 .
.
However, since the solution contains only the solute and the solvent,
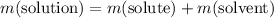 .
.
For this solution in particular,
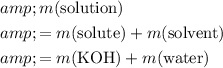 .
.
As a result,
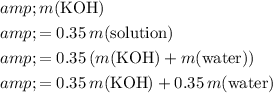 .
.
Subtract
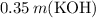 from both sides of the equation:
from both sides of the equation:
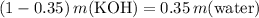 .
.
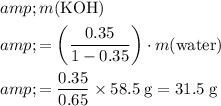 .
.
Note, that for this calculation, there's nothing special about this
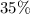 solution of
solution of
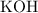 in water. In general,
in water. In general,
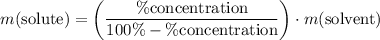 .
.