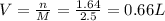Answer:
0.66 L
Step-by-step explanation:
The molarity of a solution is given by:
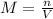 (1)
(1)
where
M is the molarity of the solution
n is the number of moles of the solute
V is the volume of the solution
In this problem we have:
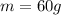 is the mass of the solute (HCl)
is the mass of the solute (HCl)
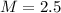 is the molarity of the solution
is the molarity of the solution
We also know that the molar mass of HCl is
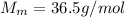
So, we can find the number of moles of HCl:
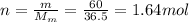
And so using eq(1), we find the volume of the solution: