50.05% is the percent yield if 17.4g of sodium hydroxide is produced when 20.0g of sodium metal reacts with 19g of water.
Step-by-step explanation:
Balanced chemical equation for the reaction:
2Na + 2
 O ⇒ 2NaOH +
O ⇒ 2NaOH +

Data given:
mass of NaOH produced = 17.4 grams
mass of Na reacted = 20 grams
mass of water reacted = 19 grams
percent yield =?
atomic mass of Na = 23 grams/mole
atomic mass of water = 18 grams/mole
atomic mass of NaOH = 39.9 grams/mole
number of moles is calculated as:
number of moles =

putting the values in above equation to know number of moles:
number of moles of Na =
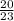
= 0.869 moles
number of moles of water =
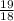
= 1.05 mole
number of moles of NaOH =
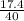
= 0.435 moles
limiting reagent in the reaction is Na
2 moles of Na reacted to give 2 moles of NaOH
So, 0.869 moles of NaOH will give 0.869 moles of NaOH
Grams of NaOH = 34.76 grams (theoretical yield)
2 moles of water will give 2 moles of NaOH
Hence, 1.05 moles will give 1.05 moles of NaOH
grams of NaOH = 42 grams
percent yield =
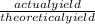
putting the values in the equation:
percent yield =
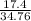 x100
x100
= 50.05 %
50.05 % is the percent yield.