Answer:
0.51g
Step-by-step explanation:
Given parameters:
Volume of nitrogen gas = 744mL
Unknown:
Mass of sodium metal produced = ?
Solution:
Let us assume that the gas, nitrogen was collected at STP which is the standard temperature and pressure, we can easily find its number of moles.
Number of moles =
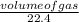
since the gas volume given is 744mL = 0.744mL
Therefore input the variables;
Number of moles =
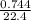 = 0.03moles
= 0.03moles
Now that we know the number of moles of the gas, let us revisit the reaction equation:
2NaN₃ → 2Na + 3N₂
It good to ensure that the reaction is balanced;
3 mole of nitrogen gas is produced with 2 moles of Na
0.03 mole of nitrogen gas will be produce with
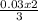 = 0.02mole
= 0.02mole
So;
Using;
mass = number of moles x molar mass
let us solve for the mass of Na;
molar mass of Na = 23g/mol
mass = 0.02 x 23 = 0.51g