1.75 moles moles of NH3 are in 0.350 L of a 5.00 M aqueous solution.
Step-by-step explanation:
Data given:
volume of the NH3 solution = 0.35 L
molarity of the aqueous solution having N
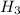 = 5 M
= 5 M
Number of moles = ?
the relation between molarity, volume and number of moles is given by the relation:
molarity =
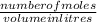
This equation is rewritten and simplified to get the number of moles is written as:
number of moles = molarity x volume in litres
putting the values in the above relation:
number of moles = 5 x 0.35
number of moles = 1.75
1.75 moles of NH3 are required to make a 5 M solution.