Answer:
C < B < A
Step-by-step explanation:
The concentration of each solution can be calculated using the formula:
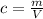
where
m is the mass of the solute
V is the volume of the solution
For solution A:
m = 4.50 g
V = 400 mL
So the concentration is
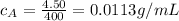
For solution B:
m = 4.50 g
V = 800 mL
So the concentration is
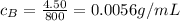
For solution C:
m = 2.25 g
V = 800 mL
So the concentration is
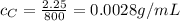
We see that
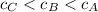
So the order from least to most concentrated is:
C < B < A