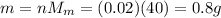Answer:
0.8 g
Step-by-step explanation:
The molarity of a solution is calculated with the following formula:
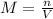
where:
n is the number of moles of the solute
V is the volume of the solution
In this problem we have:
V = 100.0 mL = 0.1 L is the volume of the solution
M = 0.20 is the molarity
So the number of moles of NaOH in the solution must be
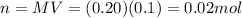
The molar mass of NaOH is
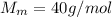
Therefore, the mass of NaOH needed in the solution is: